Facility qualification
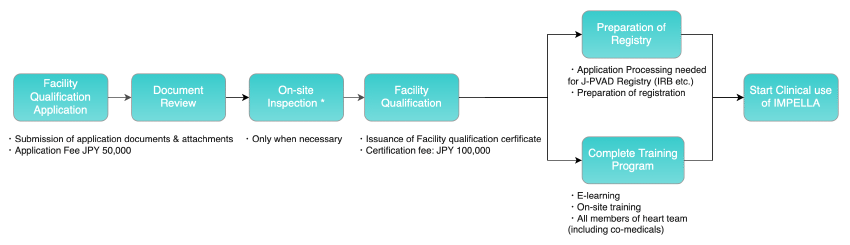
The figure below shows the process from Facility qualification through the start of clinical use of Impella at the Facility.
When medical institutions submit documents for Facility qualification, the documents will be checked, and the Council for Clinical Use of Ventricular Assist Device Related Academic Societies, Impella committee, will review the documents. The Impella Committee makes the final decision on Facility qualification based on the overall results of the document review. However, the Committee may conduct an on-site inspection to confirm the details of the application, as necessary.
The committee issues a certificate of Facility qualification which allows the manufacturer to provide the product training. The heart team and staff of the relevant departments of the certified medical institution receive and complete the training programs, after which, the devices are delivered to enable the use of Impella at the Facility.
1. Application for Facility qualification and document review
Documentation required for Facility qualification application is as follows.
Applicant Facilities fill out all of the application form documents and prepare attachments, submitting them to the Council for Clinical Use of Ventricular Assist Device Related Academic Societies, Impella committee.
Application fee: 50,000 yen
Application Documents Package
Necessary documents for Impella Facility qualification application (Excel format, Japanese language)
- Application form for Facility qualification
- Report on Facility details
- Certificate of specialists / qualified extracorporeal circulation technicians
- Letter of commitment to participate in the case Registry project
- Letter of commitment to participate in the Impella training
Applicant Facilities register their contact information, and are then given access to a web page to download the application form for Impella Facility qualification (Excel format, Japanese language).
Attachments
Copy of the receipt of bank transfer of the application fee.
Copy of the “Qualified Cardiovascular Center” certificate from the Japanese Board of Cardiovascular Surgery.
Proof of being a registered site for extracorporeal VAD treatment or qualified for implantable VAD treatment, or evidence to show the close collaboration with such sites.
※ Said proof of close collaboration shall be designated at our discretion
(please contact the committee office for details)Applicant Facility has CCU or ICU where the use of assisted circulation (IABP, PCPS) is possible.
※ Floor plan of the entire floor having CCU or ICU.Evidence to demonstrate that there are emergency / intensive care units with sufficient experience in cardiogenic shock treatment. ※ Floor plan of the entire floor where emergency and intensive care units are located.
※ Official number of cases based on the statements of medical expenses, list of actual cases, etc.Proof of the number of cardiovascular surgeries performed from January through December in the previous year.
※ Official number of cases based on the statements of medical expenses, list of actual cases, etc.Proof of the total number of IABP in the past 3 years (not required for pediatric sites)
※ Official number of cases based on the statements of medical expenses, list of actual cases, etc.Proof of the total number of PCPS/ECMO in the past 3 years.
※ Attach the case card or the list (summary of each case: implementation date, duration of implementation, etc.) with the applicant’s or representative’s signature or seal showing the official number of PCPS cases based on the statements of medical expenses, or the total number of PCPS/ECMO used in the hospital ward.Proof of the total number of PCI procedures performed in the past 3 years (not required for pediatric sites).
※ Official number of cases based on the statements of medical expenses, list of actual cases, etc.For pediatric hospitals, proof of the total number of mechanical circulatory assist (ECMO/VAD) cases for patients under 11 years old in the past 5 years.
※ Attach the case card or the list (summary of each case: implementation date, duration of implementation, etc.) with the applicant’s or representative’s signature or seal showing the official number of cases based on the statements of medical expenses, etc. or the total number of PCPS/ECMO used in the hospital ward.
Contact Information for Application Submission
Impella Committee Office
Department of Cardiovascular Surgery,
Osaka University Graduate School of Medicine and Faculty of Medicine
2-2 Yamadagaoka, Suita-shi, Osaka, 565-0871, Japan
Bank account for payment
Ibaraki Branch (Branch code: 219)
Saving account 0286563
Name: Yoshiki Sawa, The Council for Clinical Use of Ventricular Assist Device Related Academic Societies, Impella committee
2. On-site Inspection
If necessary, inspectors selected by the Council for Clinical Use of Ventricular Assist Device Related Academic Societies, Impella committee, will conduct on-site inspections.
Any travel expense related to the inspection (including accommodation fee depending on location/agenda) will be charged to the applying facility (actual fee reimbursement). The Impella Committee Office will separately inform the details of the inspection day and time, after coordinating schedules of applying medical institutions and inspectors.
3. Facility qualification
The Impella Committee makes its decision on Facility qualification based on the overall results of the document review and on-site inspection. To medical institutions that successfully acquire Facility qualification, the Impella Committee informs the medical institutions accordingly, and issues a certificate of Impella Facility qualification.
Certification fee: 100,000 yen
4. Training program
The manufacturer provides the training program.
For details, contact the manufacturer.
Facility qualification information change (for registered Facilities only)
Registered facilities with changes to the information submitted on their application form should report them immediately to the Impella Office (info@j-pvad.jp).- Change of address, contact information etc ⇒ [Change Request]
- Addition of contact ⇒ [Change Request]
- Change of person in charge ⇒ [Change Request] and [Registry Agreement]
- Change in registered professionals ⇒ [Personnel Change Request]